The Cycloset Safety Trial – overall and cardiovascular serious adverse events; glycemic control outcomes
Results Summary
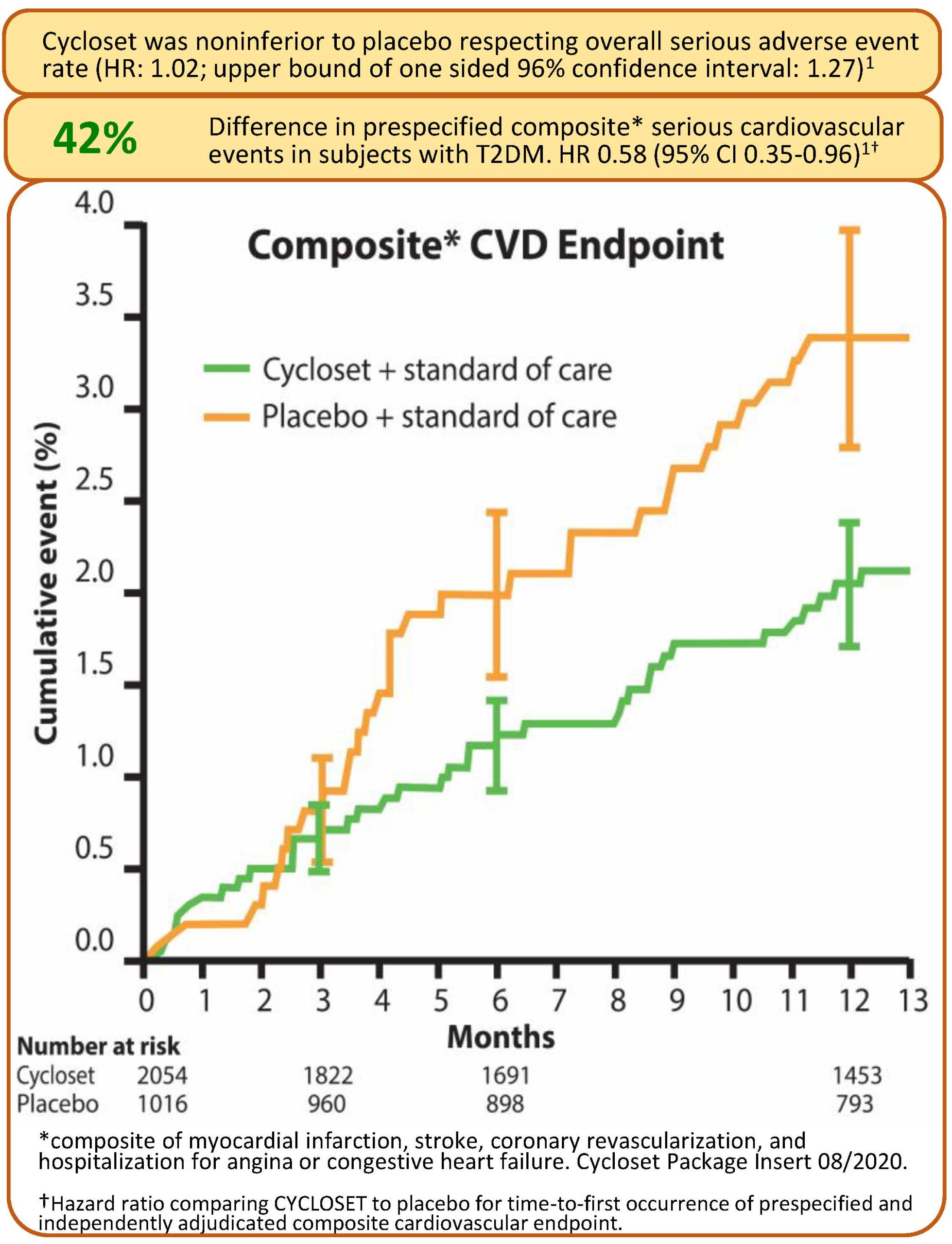
The Cycloset Safety Trial (CST) was a 52-week double-blind, placebo controlled study investigating the overall and cardiovascular serious adverse event outcomes of once-daily, circadian-timed Cycloset (1.6 to 4.8 mg/day; within 2 hours of waking in the morning) in T2DM subjects (Cycloset: N = 2054; Placebo N = 1016)
The study population had a mean baseline age of 60 years (range 27-80) and 33% were 65 years of age or older. The mean baseline body mass index was 32 kg/m2. The mean duration of diabetes at baseline was 8 years, and the mean baseline HbA1c was 7.0%. At baseline, 12% of patients were treated with diet only, 40% were treated with one oral antidiabetic agent, 33% were treated with two oral antidiabetic agents, and 16% were treated with insulin alone or insulin in combination with an oral antidiabetic agent. At baseline, 76% of patients reported a history of hypercholesterolemia, 75% reported a history of hypertension, 11% reported a history of revascularization surgery, 10% reported a history of myocardial infarction, 10% reported a history of angina, and 5% reported a history of stroke. The overall safety endpoint was time to first any serious adverse event (SAE) and the cardiovascular disease (CVD) endpoint was time to first prespecified CVD event (composite of myocardial infarction, stroke, hospitalization for heart failure, unstable angina, or revascularization surgery).
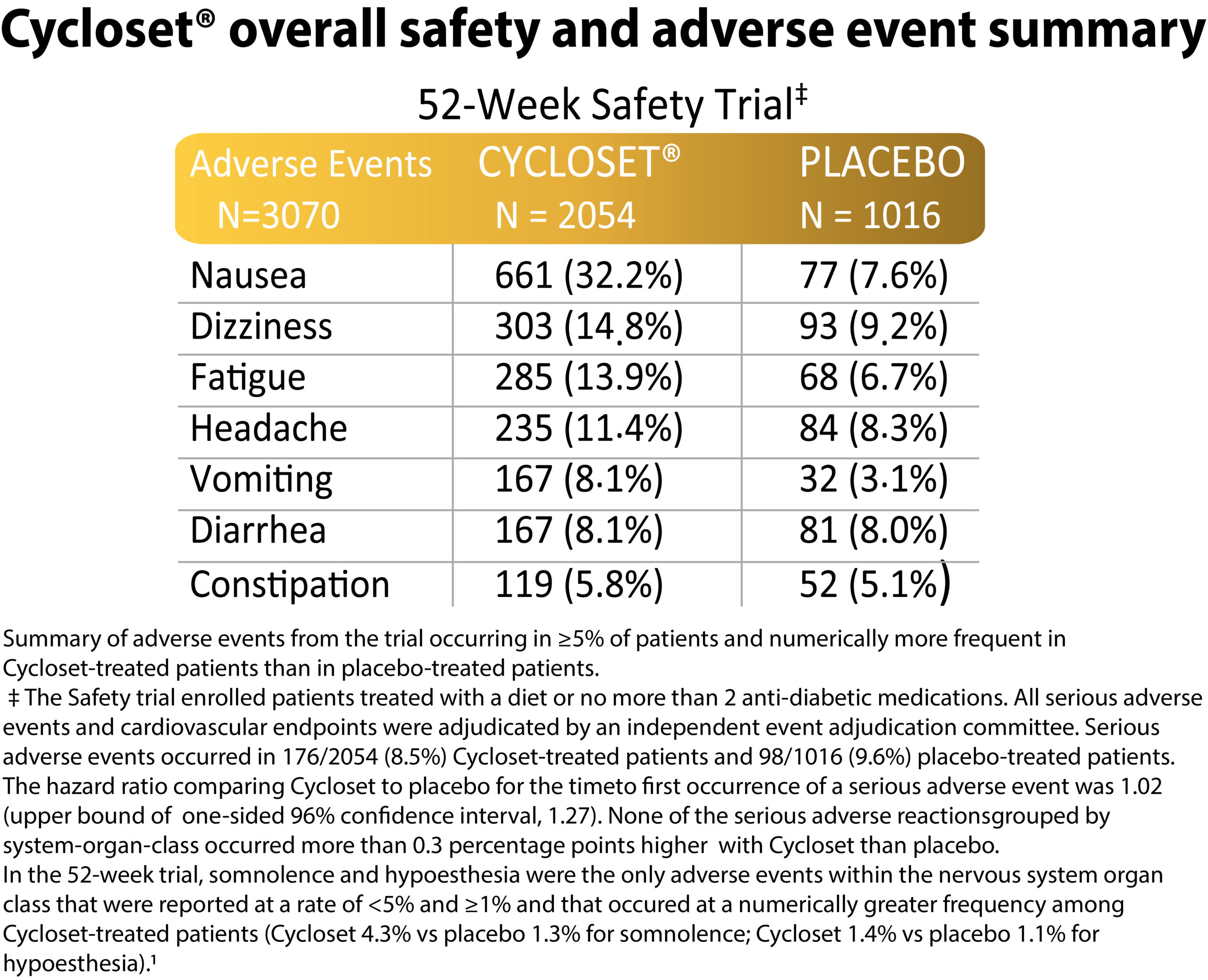
Between group differences were assessed via Cox proportional-hazards regression model. Cycloset was noninferior to placebo respecting overall serious adverse event rate (HR: 1.02; upper bound of one sided 96% confidence interval: 1.27) and reduced the risk of the CVD endpoint by 42%. (HR: 0.58; CI: 0.35 – 0.96) within the one year study. The composite cardiovascular endpoint occurred in 31 (1.5%) Cycloset-treated patients and 30 (3.0%) placebo-treated patients. Several subsequent studies were conducted investigating the Cycloset impact on CVD outcome within various subpopulations of the CST and also investigating the drug’s impact on neuroendocrine and proinflammatory pathologies known to precipitate CVD in the T2DM population and have been published in the peer reviewed scientific literature.
CONTRAINDICATIONS Hypersensitivity to ergot-related drugs, bromocriptine or to any of the excipients in CYCLOSET. History of syncopal migraines. Postpartum patients. Lactating patients.
WARNINGS AND PRECAUTIONS Hypotension: Can cause orthostatic hypotension and syncope, particularly upon initiation or dose escalation. Use caution in patients taking antihypertensive medications. Assess orthostatic vital signs prior to initiation of CYCLOSET and periodically thereafter. Advise patients during early treatment to avoid situations that could lead to injury if syncope was to occur. Psychosis: May exacerbate psychotic disorders or reduce the effectiveness of drugs that treat psychosis. Use in patients with severe psychotic disorders is not recommended. Impulse control/compulsive behaviors: Ask patients or their caregivers about new or increased gambling urges, sexual urges, uncontrolled spending, or other urges while being treated with CYCLOSET. Consider dose reduction or stopping CYCLOSET if a patient develops such urges. Use of CYCLOSET in patients with impulse control/compulsive behaviors is not recommended. Somnolence: May cause somnolence. Advise patients not to operate heavy machinery if symptoms of somnolence occur. Interaction with dopamine antagonists: Concomitant use with dopamine antagonists such as neuroleptic agents may diminish the effectiveness of both drugs. Concomitant use is not recommended. Other dopamine receptor agonists: Effectiveness and safety are unknown in patients already taking dopamine receptor agonists for other indications. Concomitant use is not recommended. Risks in Postpartum Patients: Serious and life-threatening adverse reactions have been reported.
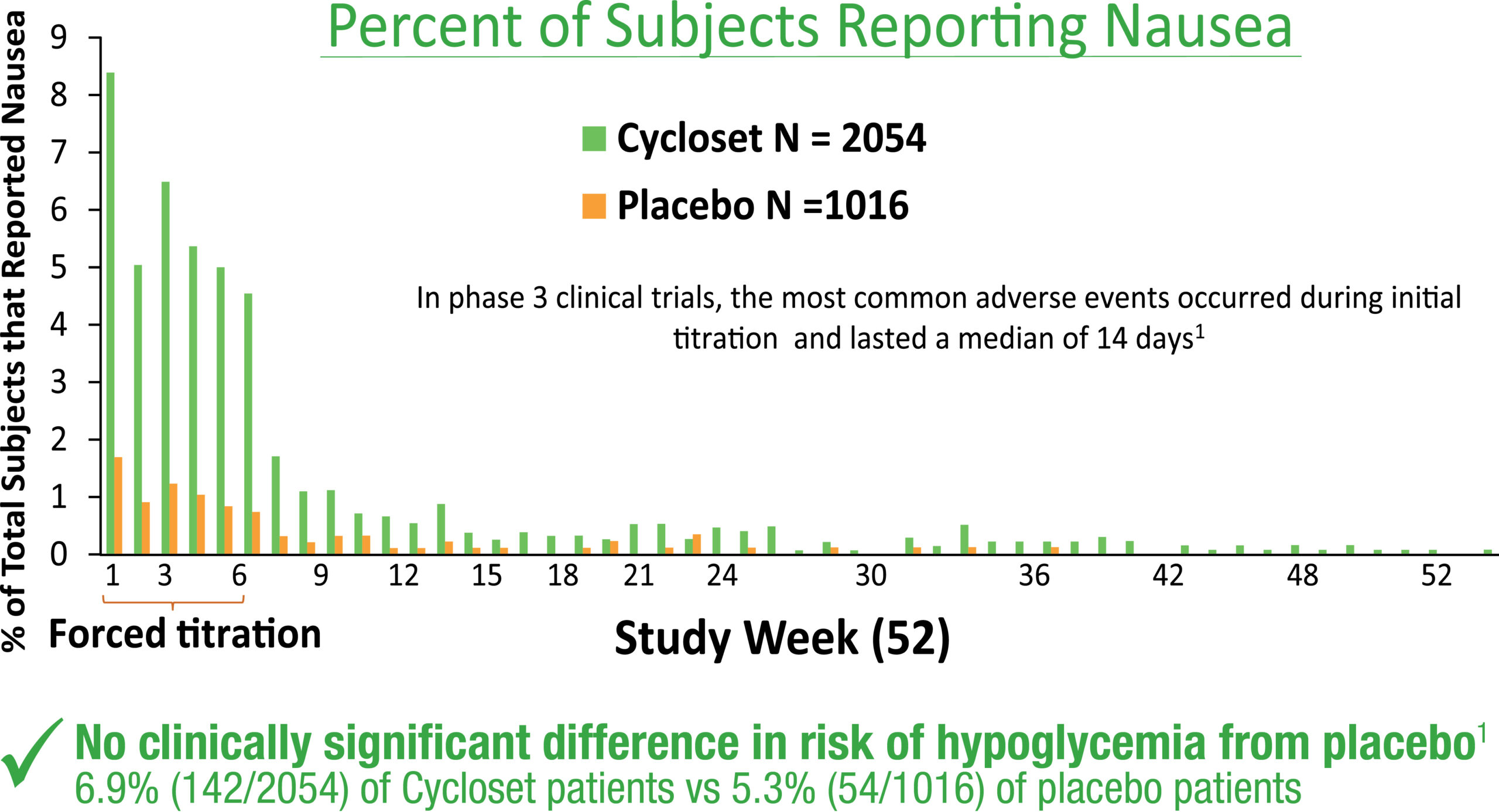
ADVERSE REACTIONS In controlled clinical trials, adverse reactions reported in ≥5% of patients treated with CYCLOSET and reported more commonly than in patients treated with placebo, included nausea, fatigue, dizziness, vomiting, and headache. Postmarketing reports with higher doses of bromocriptine used for other indications include psychotic disorders, hallucinations, and fibrotic complications.
- References, Cycloset package insert and peer-reviewed publications:
- CYCLOSET [PACKAGE INSERT]. Tiverton, RI: VeroScience LLC; 2020
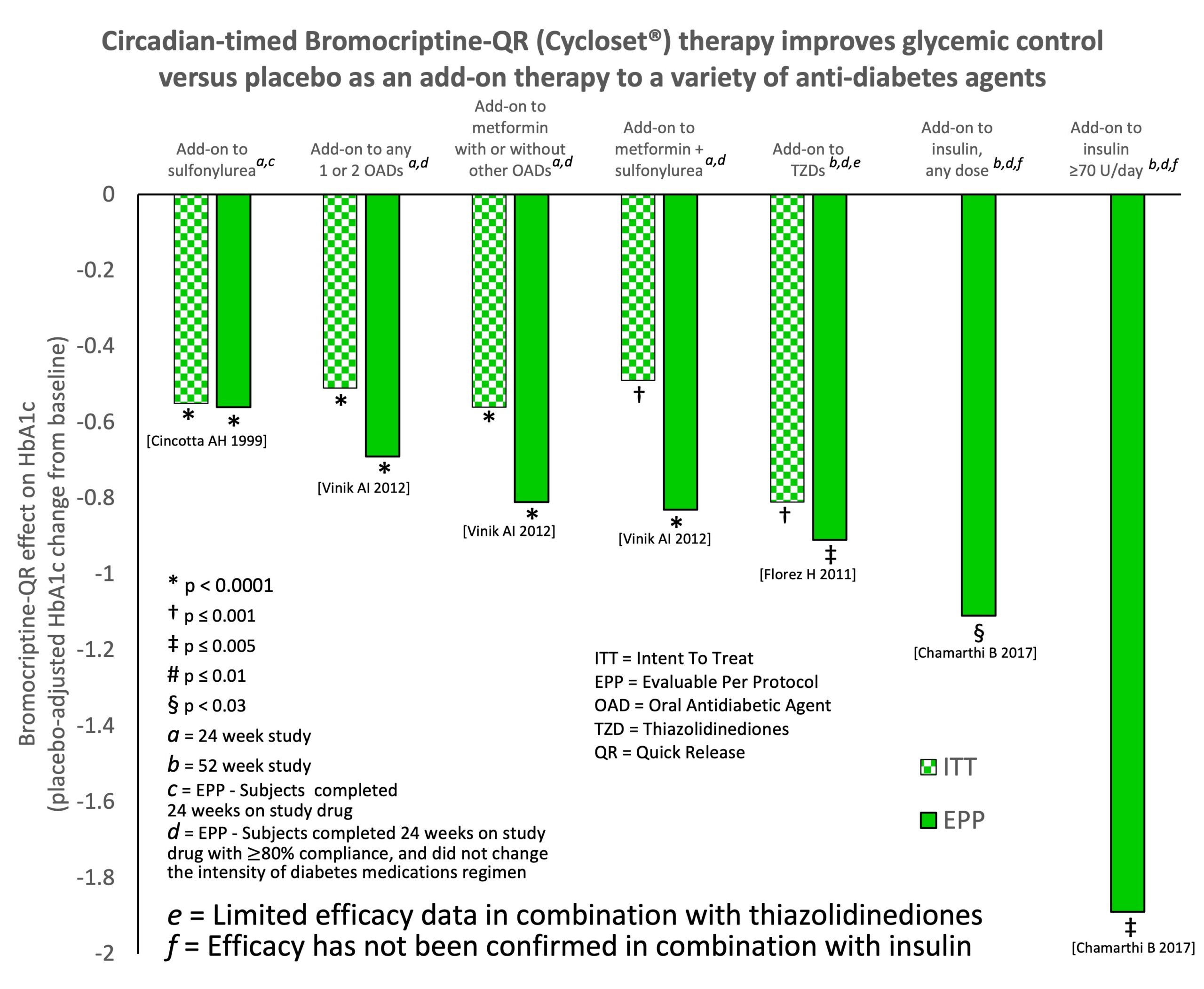
- Cincotta, H.; Meier, A.H.; Cincotta, M., Jr. Bromocriptine improves glycaemic control and serum lipid profile in obese Type 2 diabetic subjects: A new approach in the treatment of diabetes. Expert Opin. Investig. Drugs 1999, 8, 1683–1707.
- Vinik, I.; Cincotta, A.H.; Scranton, R.E.; Bohannon, N.; Ezrokhi, M.; Gaziano, J.M. Effect of Bromocriptine-Qr On Glycemic Control in Subjects with Uncontrolled Hyperglycemia On One or Two Oral Anti-Diabetes Agents. Endocr. Pract. 2012, 18, 931–943.
- Florez, H.; Scranton, R.; Farwell, W.R.; DeFronzo, R.A.; Ezrokhi, M.; Gaziano, J.M.; Cincotta, A.H. Randomized Clinical Trial Assessing the Efficacy and Safety of Bromocriptine-QR when Added to Ongoing Thiazolidinedione Therapy in Patients with Type 2 Diabetes Mellitus. J. Diabetes Metab. 2011, 2, 142.
- Chamarthi, B.; Cincotta, A.H. Effect of bromocriptine-QR therapy on glycemic control in subjects with type 2 diabetes mellitus whose dysglycemia is inadequately controlled on insulin. Postgrad. Med. 2017, 129, 446–455.